Back
Nimesh Pinnamaneni
•
Helixworks Technologies • 12m
Why qPCR & Phenotypic Testing Must Be Displaced? – Part III This is the third part of a deep dive into why qPCR & phenotypic testing need to be replaced. In Part I, I covered the limitations of current diagnostics & why incremental improvements aren’t enough. Part II looked at targeted Next-Gen Sequencing (tNGS) as a potential solution—capable of identifying both pathogens & resistance in a single test. But if tNGS is going to work in clinical settings, it needs to be as simple as qPCR. Right now, it’s not. The biggest obstacle isn’t sequencing—it’s automation. That’s what I’m tackling in Part III. The Challenge of Automating Sequencing for Clinical Use If you need molecular diagnostics in a hospital, you use qPCR. It’s fast, simple, & has been the standard for decades. Cepheid has been around for 26 years, with 40,000 GeneXpert instruments installed & $2 billion in revenue. Their system works because it’s designed for clinicians, not lab scientists—drop in a sample, press a button, & get results. No complex prep, no custom protocols. Cepheid isn’t the only player anymore. Indian company Molbio Diagnostics has taken a similar approach. Their Truenat system is a real-time RT-PCR platform designed for low-resource settings. It’s already deployed in ~8,000 hospitals & clinics globally. More importantly, the cost per test is competitive—$7.90 per sample, similar to Cepheid’s $8 cartridges. It’s clear that automation works when the workflow is simple. Truenat, like GeneXpert, is built around one test, one result, one action. No batching, no custom protocols, no flexibility. That’s why it succeeds in clinical settings. Why Isn’t Sequencing as Simple as qPCR? Sequencing is messy. It’s not just about running a machine—it’s about sample prep, library prep, sequencing, & analysis. Each step requires specialised reagents, workflows, and trained personnel. That’s why sequencing is still mostly confined to research labs & high-end clinical centres. It’s powerful, but not simple. People have tried to automate sequencing workflows. Pipette robots are one approach. These are flexible and can automate parts of the process, but they require maintenance, trained staff, and custom protocols. They don’t eliminate complexity—they just shift it. Instead of pipetting by hand, you program the robot to pipette for you. Full automation still isn’t plug-and-play. Another approach is closed-box automation—machines like MagicPrep NGS that handle everything for you, as long as you use their kits and follow their protocol. It works, but locks users into fixed workflows. Need to change anything—multiplexing, target selection? You’re out of luck. Then there are digital fluidics platforms like Illumina’s NeoPrep or ONT’s Voltrax. These miniaturise reactions into a microfluidic chip—elegant in theory but expensive and rarely adopted. One of the newest attempts to tackle this problem is Volta Labs’ Callisto System. Instead of pipettes or cartridges, Callisto uses electrowetting-based digital fluidics—tiny droplets move around a chip, performing DNA extraction and library preparation without physical pipetting. It’s a brilliant idea. But it’s still a $125,000 benchtop instrument, which makes it out of reach for most hospitals and small labs. It’s automation, but not yet clinical automation. Meanwhile, Cepheid and Molbio still dominate clinical molecular diagnostics with their simple, cartridge-based qPCR systems. Why Not Just Build a Sequencing Cartridge Like Cepheid? Technically, there’s nothing stopping someone from making a cheap, plastic, preloaded reagent cartridge that automates sequencing prep. But the problem isn’t technical—it’s demand. The whole reason sequencing is powerful is its flexibility. Labs want to run different assays, multiplex samples, and tweak protocols. A single-use cartridge locks them into a fixed workflow they don’t want. That’s why no one has built a sequencing equivalent of Cepheid’s GeneXpert—it would be a niche product at best. The real problem isn’t the lack of automation. It’s that sequencing users don’t want the kind of automation qPCR users need. Where Does That Leave Us? There’s still room for improvement. The transition from manual prep to automated sequencing isn’t fully solved. The right solution probably isn’t a single cartridge system but something modular—automating the most painful parts while keeping flexibility where it’s needed. Maybe that means hybrid systems that allow some customisation. Maybe it means smarter automation that adapts protocols based on sample type. But if we’re looking for a one-size-fits-all, push-button sequencing solution, we may be searching for something that clinicians don’t actually want, and sequencing labs don’t actually need. That’s why automation in sequencing isn’t a solved problem. Not because it’s too hard, but because it’s not clear what problem needs to be solved. Let’s Talk. Drop a comment or DM me—let’s figure this out together. 🚀
More like this
Recommendations from Medial
Nimesh Pinnamaneni
•
Helixworks Technologies • 11m
Looking to connect with folks in clinical diagnostics and molecular biology! I’m building a new clinical lab focused on infectious disease diagnostics powered by DNA sequencing — and would love to connect with people who have experience in: • Runni
See MoreNimesh Pinnamaneni
•
Helixworks Technologies • 12m
Why qPCR & Phenotypic Testing Must Be Displaced – Part II If you ever want to understand the scale of a problem, look at how much it costs. Tuberculosis alone costs India, Africa, and Brazil 27 trillion INR a year in lost productivity and healthcare
See More
Account Deleted
Hey I am on Medial • 5m
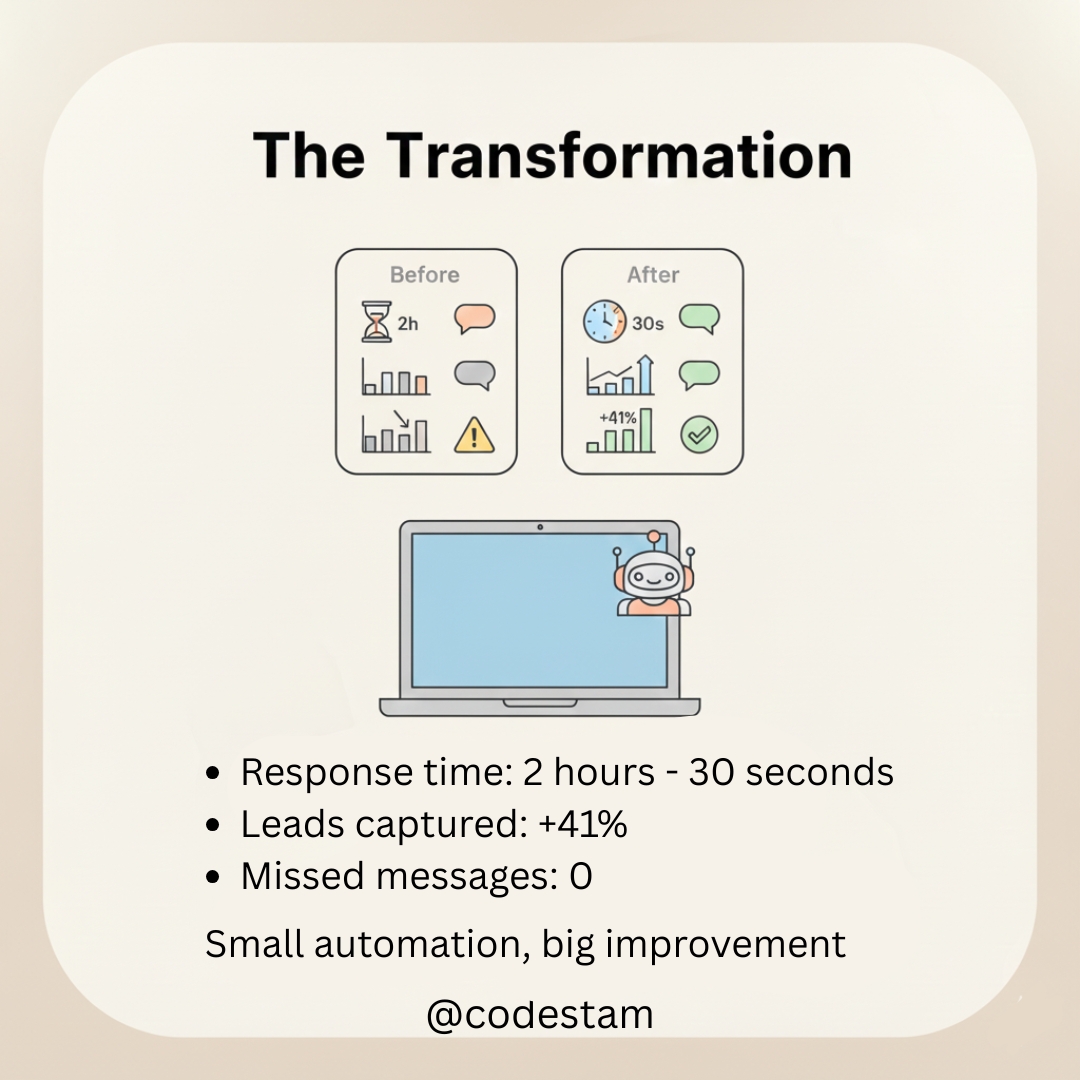
Every founder dreams of scaling fast. But here’s the truth → scaling without systems is chaos. That’s why I’m obsessed with AI automation. Imagine: ✅ Your clients get replies instantly ✅ Reports auto-generate every day ✅ Sales follow-ups run while
See MoreVasvi Seth
Cyber Security Stude... • 1y
Decoding Network Protocols: Communication, Management, and Security Network protocols are the backbone of our digital world, enabling seamless communication between devices. These protocols can be broadly categorized into three main types: communica
See MoreNimesh Pinnamaneni
•
Helixworks Technologies • 1y
Can Sequencing replace traditional diagnostics in India? Traditional diagnostics are slow. Culture and antibiotic resistance tests take days or even weeks to deliver results. This can be detrimental for patients suffering from sepsis or fever of unk
See MoreAccount Deleted
Hey I am on Medial • 7m
AI isn’t the future — it’s already transforming businesses today. At Opslify Software Solution, we help you unlock growth with: ✅ AI-powered Web Apps ✅ Smart Business Automation ✅ Custom AI Applications ✅ Intelligent Design Solutions Whether you're
See MoreAccount Deleted
Hey I am on Medial • 4m
AI isn’t the future — it’s already transforming businesses today. At Opslify Software Solution, we help you unlock growth with: ✅ AI-powered Web Apps ✅ Smart Business Automation ✅ Custom AI Applications ✅ Intelligent Design Solutions Whether you're
See MoreDownload the medial app to read full posts, comements and news.










/entrackr/media/post_attachments/wp-content/uploads/2021/08/Accel-1.jpg)





















