Back
Anonymous 1
Hey I am on Medial • 11m
10x far better than USA. You know what's the issue with generic medicine. Quality Concerns & Regulatory Issues Many small manufacturers do not meet strict Good Manufacturing Practices (GMP), leading to variations in drug efficacy and safety. The Central Drugs Standard Control Organization (CDSCO) struggles with strict enforcement, leading to substandard or counterfeit drugs entering the market. The US FDA and EU have flagged multiple Indian pharma plants for violations, affecting trust in Indian generics globally.
Replies (1)
More like this
Recommendations from Medial
My Dawai Wala
India's Health First... • 8m
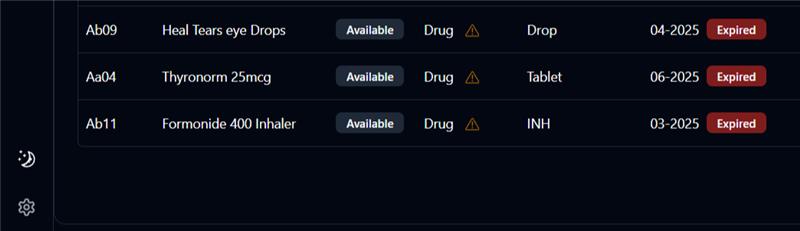
Day #7 Real Medicine. Not Maybes. Let’s talk about a silent threat in Indian healthcare: • Fake pills • Near-expiry stock • Unexplained substitutions This isn’t rare. It’s real and it’s happening every day. • Counterfeit drugs make up 8–10% of Ind
See More
Aakash kashyap
Building JalSeva and... • 1y
❗❗Important For All ❗❗ A $450 million Indian startup, Zest Money, shut down due to strict RBI regulations, highlighting the risks of external factors in business. 🚀 $450 million valuation: Zest Money was a leading player in the buy now pay later s
See More
Download the medial app to read full posts, comements and news.








/entrackr/media/post_attachments/wp-content/uploads/2021/08/Accel-1.jpg)





















